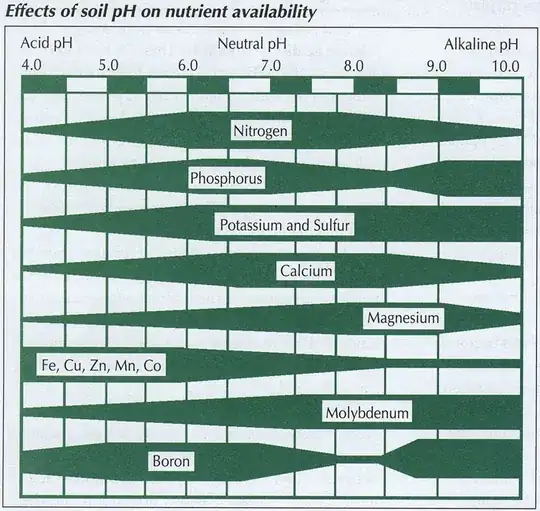
As Why do blueberries need acidic soil? points out, it seems that iron is really the biggest nutrient to suffer in alkaline conditions. Even an answer there, despite a nice emphasis on the broader soil ecology, ends up focusing on this nutrient:
Acidic soil … to release phosphates, iron, magnesium which your blueberries love. The iron particularly is needed …
Similarly an answer to Is it really important to give blueberry plants acidic soil? quickly introduces a similar claim:
At higher pH, the bushes will have an iron deficiency…
So as much as I appreciate building good soil, I've seen doubts cast that I'll really be able to pull it off to the extent blueberries theoretically need. (E.g. "pine needles aren't really acidic", "sulfur discourages worms and/or mycorrhizal fungi from thriving", "all your irrigation water is going to be alkaline anyway"… I'm not particularly intending to debate those claims here.)
I've got some chelated iron around (HEDTA if memory serves) that I use in my aquaponics systems. It is iron bound to a "helper" molecule such that [they claim] the plant can take in the iron and use it, at much higher pH levels than where iron is normally available.
What would happen if I simply gave my blueberries an occasional foliar application of iron, or periodically top dressed the soil with some of the powder, and didn't worry so much about changing the actual pH of the soil?