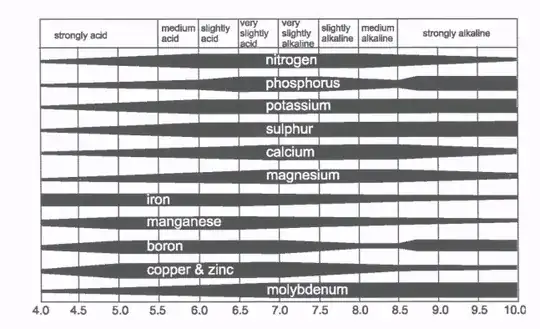
I know that blueberries need soil in the range of ph 4-5, but no one ever says why they need the pH in that range. I've heard that it may be because of a lack of available iron at higher pH, and that other plants emit acidic in their root zone to create iron that they can use. So is it all about the iron? This chart of nutrients avaiable by pH is also interesting...it looks like the only nutrient available at low pH is iron. But if iron is the limiting factor, it looks like it is available up to pH 6.5. So why the pH 4-5?
- 275
- 2
- 8
1 Answers
Blueberries have a rudimentary root system that doesn't have the finer root hairs found on most plants.
They grow best in forest duff (lots of acidic, organic matter). The acidic nature of the soil causes bacteria and fungi to thrive that release minerals and ammonia that blueberry bushes thrive on and that their roots can readily absorb.
In plant root systems it's more about symbiosis and the nutrient pool it creates than the measurable byproducts (acidity, nitrogen, etc.). The main difference between woodlands and gardens is the method of nitrogen uptake (ammonia vs. nitrates).
Neutral to very slightly acidic soil tends to be a nitrate rich source of nitrogen, with mostly bacterial activity (the rest of your garden), whereas acidic soil favors more fungal activity and an ammonia rich environment for the nitrogen source. Acidic soil also hosts acid loving bacteria (which that chart doesn't address) that directly attack mineral in the soil to release phosphates, iron, magnesium which your blueberries love. The iron particularly is needed to prevent chlorosis so the leaves can create enough chlorophyll.
- 3,963
- 20
- 20